Molecular Landscapes by David S. Goodsell
CytoSkeleton, 2021
Acknowledgement: Illustration by David S. Goodsell, RCSB Protein Data Bank. doi: 10.2210/rcsb_pdb/goodsell-gallery-036
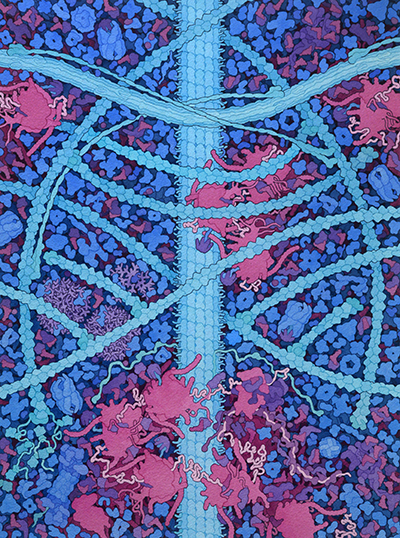
This painting is a conceptual image of the cytoplasm of a human cell. Three types of cytoskeletal filaments are shown: (A) actin, (B) intermediate filament, and (C) microtubule. The end of one actin filament is being extended with the help of (D) formin, and other filaments have (E) capping protein at their ends. Junctions linking actin filaments are formed by (F) Arp2/3, and actin and the intermediate filament are linked by (G) plectin. The soluble biomolecules filling the spaces around the cytoskeletal filaments include the 100 most abundant proteins from a quantitative proteomics study (reference 8 below). Several features represent substantial changes from previous paintings: (H) ribosome structures include long RNA expansion segments and intrinsically-disordered stalk proteins that recruit tRNA and elongation factors, and (I) nine aminoacyl-tRNA synthetases are associated into a large multi-tRNA-synthetase complex.

Selected References
1. Chakraborty, S., Jasnin, M., and Baumeister, W. (2020) Three‐dimensional organization of the cytoskeleton: A cryo‐electron tomography perspective. Protein Sci. 29, 1302–1320
2. Pollard, T. D. (2016) Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 8, a018226
3. Merino, F., Pospich, S., and Raunser, S. (2020) Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin. Cell Dev. Biol. 102, 51–64
4. Parry, D. A. D., Strelkov, S. V., Burkhard, P., Aebi, U., and Herrmann, H. (2007) Towards a molecular description of intermediate filament structure and assembly. Exp. Cell Res. 313, 2204–2216
5. Eldirany, S. A., Lomakin, I. B., Ho, M., and Bunick, C. G. (2021) Recent insight into intermediate filament structure. Curr. Opin. Cell Biol. 68, 132–143
6. Janke, C., and Magiera, M. M. (2020) The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326
7. Janda, L., Damborský, J., Rezniczek, G. A., and Wiche, G. (2001) Plectin repeats and modules: strategic cysteines and their presumed impact on cytolinker functions. BioEssays News Rev. Mol. Cell. Dev. Biol. 23, 1064–1069
8. Beck, M., Schmidt, A., Malmstroem, J., Claassen, M., Ori, A., Szymborska, A., Herzog, F., Rinner, O., Ellenberg, J., and Aebersold, R. (2011) The quantitative proteome of a human cell line. Mol. Syst. Biol. 7, 549
9. Weisser, M., and Ban, N. (2019) Extensions, extra factors, and extreme complexity: Ribosomal structures provide insights into eukaryotic translation. Cold Spring Harb. Perspect. Biol. 11, a032367
10. Liljas, A., and Sanyal, S. (2018) The enigmatic ribosomal stalk. Q. Rev. Biophys. 51, e12
11. Khan, K., Baleanu-Gogonea, C., Willard, B., Gogonea, V., and Fox, P. L. (2020) 3-Dimensional architecture of the human multi-tRNA synthetase complex. Nucleic Acids Res. 48, 8740–8754